02 Jul, 2025
Biological Indicators (BIs) are critical tools for validating sterilization processes. Their core component is specific types of bacterial spores, selected for their extreme resistance to sterilization conditions. These spores simulate the hardest-to-kill microorganisms, ensuring the effectiveness of sterilization. Below are the common bacteria used in BIs and their applications:
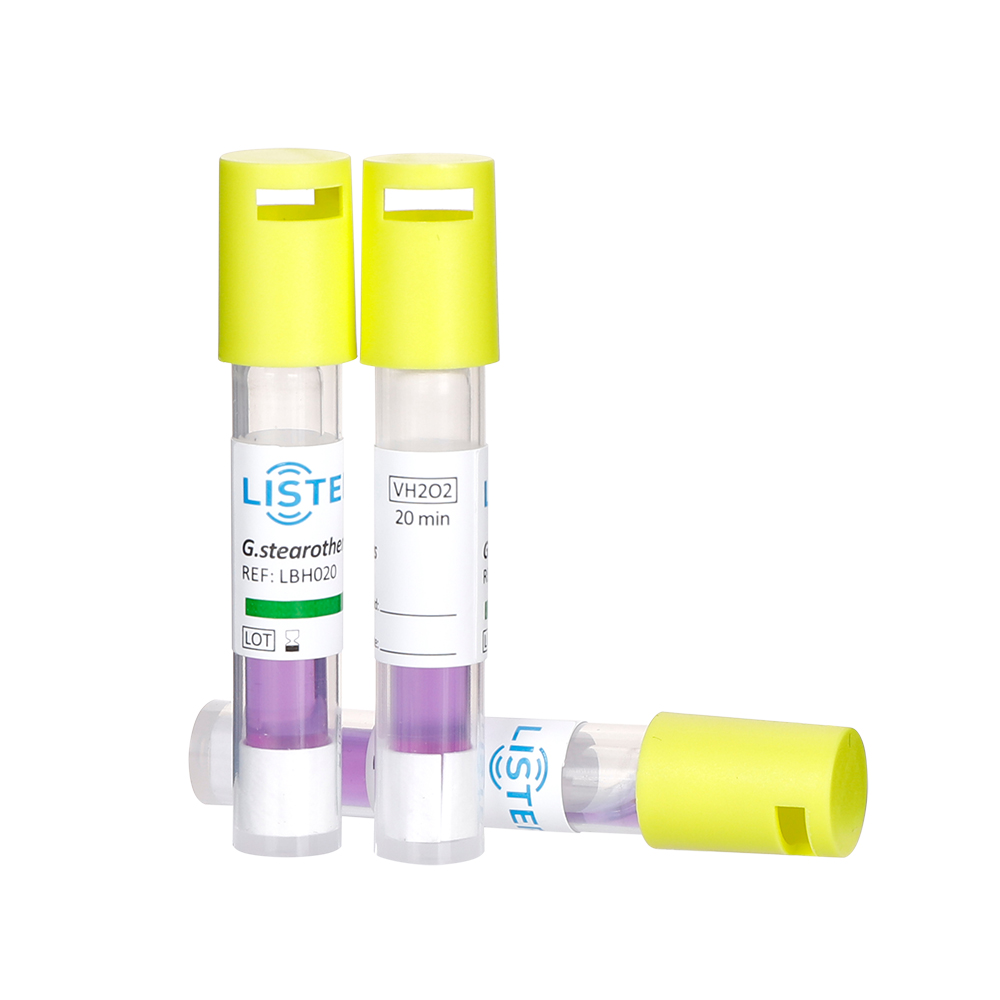
1. Geobacillus stearothermophilus
Characteristics: A highly heat-resistant, gram-positive bacterium. Its spores can survive high-temperature steam environments (e.g., above 121°C).
Applications: Primarily used to validate steam sterilization (e.g., autoclaves) and hydrogen peroxide gas plasma sterilization.
Detection: Successful sterilization is confirmed if spores are inactivated, leaving culture media unchanged (typically purple).
2. Bacillus atrophaeus
Characteristics: Exhibits strong resistance to dry heat and chemical sterilants like ethylene oxide (EO).
Applications: Validates ethylene oxide sterilization (EO) and dry heat sterilization (e.g., ovens above 160°C).
Industries: Medical devices, pharmaceutical equipment, and laboratory tools.
3. Clostridium sporogenes
Characteristics: An anaerobic bacterium with heat-resistant spores capable of surviving oxygen-free environments.
Applications: Used in specialized sterilization processes (e.g., supplemental validation for moist heat sterilization) or food industry sterilization monitoring.

Why Are These Bacteria Chosen?
Spore Resistance: Their multi-layered spore structures (core, cortex, coat) protect against extreme conditions.
Standardization: International standards (e.g., ISO 11138, ISO 11135) mandate specific strains for consistent results.
Safety: selected strains are non-pathogenic, minimizing operational risks.
How Do Biological Indicators Work?
Pre-sterilization: Spore-loaded carriers (e.g., strips, vials) are placed inside the sterilizer.
Post-sterilization: Carriers are incubated under controlled conditions (at specific temperatures).
Result Interpretation: No microbial growth (e.g., no color change to yellow) indicates successful sterilization.
Applications
Healthcare: Validating sterilization of surgical instruments and implants.
Pharmaceuticals: Compliance checks for sterile production lines and filling equipment.
Laboratories: Quality control for sterilized media and reagents.
Conclusion
LISTER Biological Indicators, standardized with resilient bacterial spores, serve as the "gold standard" for sterilization assurance. Understanding their core bacteria and mechanisms helps industries optimize sterilization protocols, mitigate contamination risks, and ensure product and patient safety.

Tags: