02 Jul, 2025
Steam sterilization, commonly known as autoclaving, is a cornerstone of infection control in healthcare, pharmaceuticals, and laboratory settings. While physical and chemical monitoring methods (e.g., temperature, pressure, and indicator strips) provide real-time data, biological indicators (BIs) remain the gold standard for validating the efficacy of sterilization cycles. These living test systems offer direct evidence that the process can inactivate even the most resistant microorganisms.

How Do They Work?
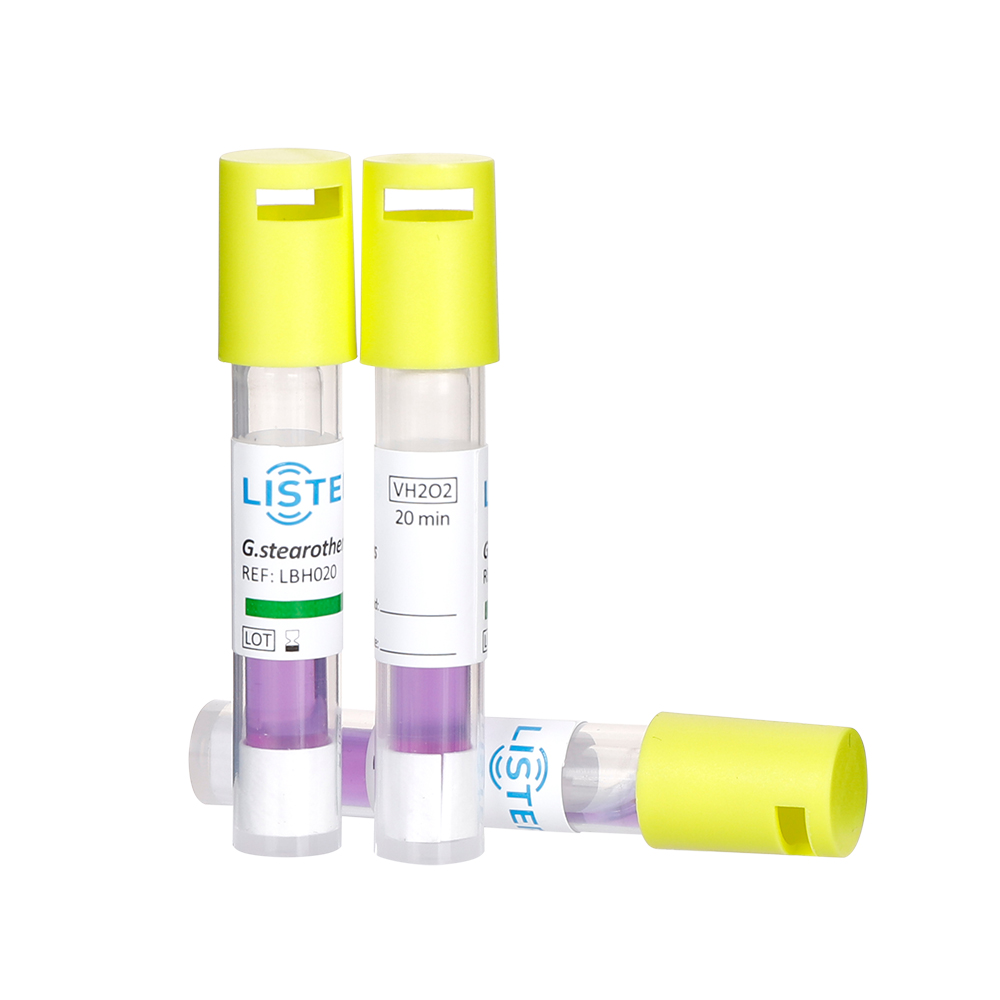
After exposure to a sterilization cycle, BIs are incubated under controlled conditions. If the sterilization process was effective, no bacterial growth will occur. Conversely, growth indicates a failure, prompting investigation into potential causes such as equipment malfunction, improper loading, or procedural errors. Modern BIs often include self-contained vials with growth media, streamlining incubation and reducing contamination risks.
Regulatory Standards and Applications
Biological indicators must comply with international standards (e.g., ISO 11138-1:2017) to ensure consistency and reliability. They are indispensable for routine validation, requalification of sterilizers, and critical applications like implantable device processing. Unlike chemical indicators, which only confirm exposure to specific conditions, BIs provide a direct measure of microbial lethality.
Conclusion
Biological indicators bridge the gap between theoretical sterilization parameters and real-world microbial inactivation. By verifying that even the most resistant organisms are eradicated, they safeguard patient safety and uphold the integrity of sterile products. As sterilization technologies evolve, BIs will continue to play a vital role in quality assurance across industries.
LISTER is committed to Present Scientific BI + CI + PCD Integrated Solutions for Sterilization. Our customers span across Europe, the Middle East, South America, and Southeast Asia, and in the future, more people will use and recognize our biological idncators.
Our portfolio includes Biological Indicators(BIs), Chemical Indicators(CIs), Rapid Biological Auto-reader Incubators ,Process challenge device(PCD) and Bowie-Dick Test Packs, that are designed to meet the stringent demands of medical device sterilization and ensure compliance with global safety standards.

Tags: